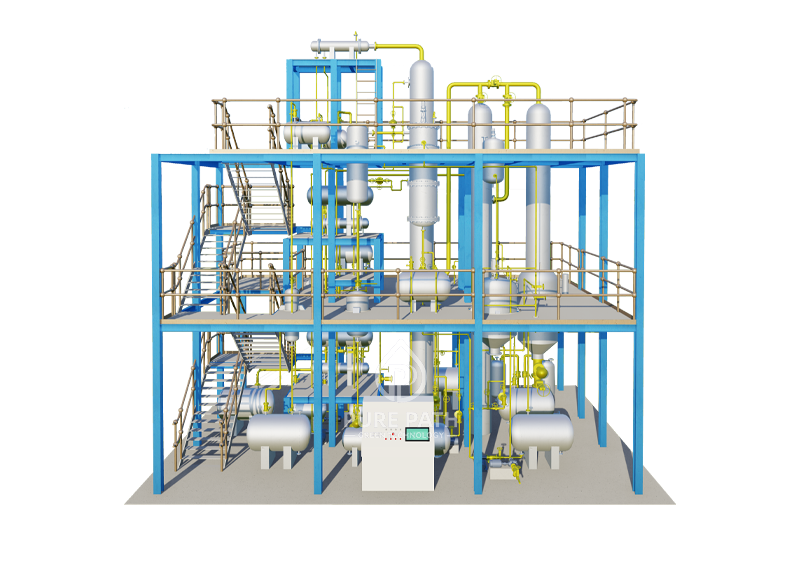
GENUINE CONTINUOUS WASTE LUBRICATING OIL REFINERY FOR SMALL TO MEDIUM SCALE PROJECT
Waste Oil to Diesel Plant
PPGT-DF Waste Oil Distillation Plant is
designed to crack the long chain
hydrocarbons of waste motor oil into
valuable diesel fuel, and some byproducts, such as light hydrocarbons, water and asphaltene sludge. With PurePath
exclusive fractionating and ultrasonic
desulfuring technologies, the diesel which is
produced by our distillation plant is able to
meet Euro-V related standards.
Waste Oil to Base oil Plant
PurePath has redesigned the re-refining
technologies for used lubricating oil to
produce near virgin quality base oil ranging
from SN80 to SN500 by using different
technolologies Such as full continuous
distillation, wiped film evaporation and short
path distillation. In the meanwhile, we have
aslo developed base oil solvent extraction
plant to help you to get rid of annoying clay
refining system and allow you to refine the
base oil to meet API group 1+ standards.
Industrial Solutions
PurePath Wisdom Turning Waste Into Gold.
PurePath Strength
15
15 years experiences in Petrochemical equipment industry.
40
Built up over 40 refineries all over the world.
5000
Over 5,000sqm of modernized workshop
50
More than 50 exclusive technologies & innovitions.
PUREPATH STRENGTH
Service You Can Rely On
PurePath is committed to providing our customer with exclusive turnkey solution and extraordinary services all over the pre-sales, manaufacturing, delivery, installation and after sales process to make sure that each of our customer will be satisfied to our services and products.
PUREPATH FOR A PURE PLANET
PurePath has implemented 5S site management in the modernized workshop. With the advanced production facilities & quality control system, skilled workers. PurePath is able to manufacture over 20 sets of state-of-of-art petrochemical plant annually.
PurePath is now becoming a shining star who is combining the research, development, engineering & manufacturing in petrochemical equipment industry. To devoting ourselves for a Pure Planet is the biggest mission of PurePath, for which we have been fulfilling for more than 14 years.