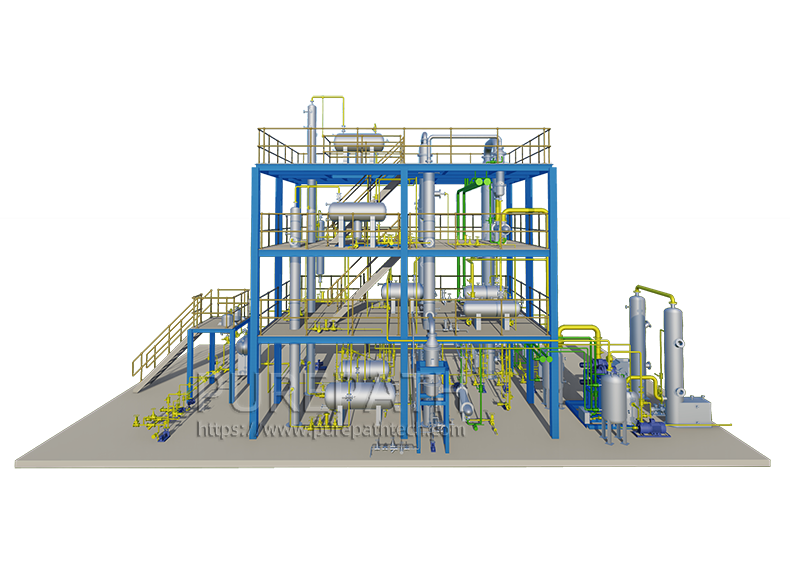
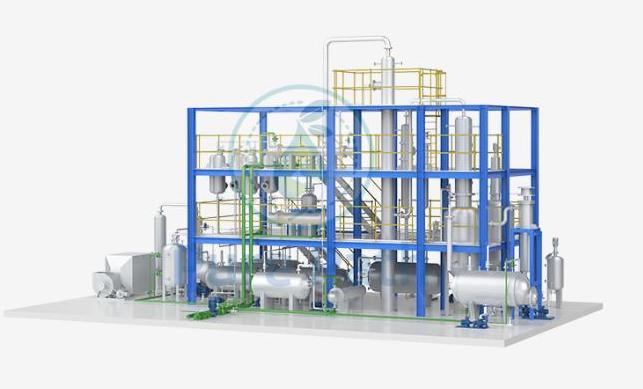
Diesel Desulfurization: Paving the Way for Cleaner Fuel and a Greener Future
Diesel fuel is a vital energy source that powers various sectors of our economy, from transportation to industry. However, one of the major drawbacks of diesel fuel is its sulfur content, which contributes to harmful emissions and environmental pollution. To combat this issue, diesel desulfurization processes have been developed to reduce the sulfur content in diesel fuel. In this article, we will delve into the various aspects of diesel desulfurization, including the types of processes involved, the underlying chemistry, the catalytic role in the process, and the significant environmental benefits it offers.

Types of Diesel Desulfurization Processes
Hydrodesulfurization (HDS)
Hydrodesulfurization is the most common and widely used method for reducing sulfur content in diesel fuel. This process involves subjecting diesel fuel to high-pressure and high-temperature conditions in the presence of hydrogen gas and a catalyst, typically composed of metals like cobalt and molybdenum. During HDS, the sulfur compounds in the diesel fuel react with hydrogen gas to form hydrogen sulfide (H2S), which is then removed, resulting in low-sulfur diesel fuel.
Oxidative Desulfurization (ODS)
Oxidative desulfurization is an alternative method that operates at lower temperatures compared to HDS. In ODS, diesel fuel is treated with oxidizing agents, which oxidize sulfur compounds into sulfoxides and sulfones. These oxidized sulfur compounds can be more easily removed from the fuel, leaving behind low-sulfur diesel. ODS is advantageous because it consumes less energy compared to HDS.
Adsorptive Desulfurization
Adsorptive desulfurization relies on the use of solid adsorbents to selectively adsorb sulfur compounds from diesel fuel. The adsorbents have a high affinity for sulfur-containing molecules, allowing them to capture sulfur compounds as the fuel passes through a bed of adsorbent material. Once saturated with sulfur, the adsorbents can be regenerated for reuse. This method is effective for removing even trace amounts of sulfur.
Chemistry of Diesel Desulfurization
A. Hydrodesulfurization Chemistry
In HDS, the chemistry revolves around the reaction between sulfur-containing compounds in diesel fuel and hydrogen gas in the presence of a catalyst. The catalyst, typically composed of cobalt and molybdenum, facilitates the breaking of sulfur-carbon bonds. Sulfur compounds are converted into hydrogen sulfide (H2S), which can be easily separated from the fuel. The overall reaction can be summarized as follows:
R-S-R’ + 3H2 → 2RH + H2S
Where R and R’ represent hydrocarbon groups in the sulfur compounds.
B. Oxidative Desulfurization Chemistry
Oxidative desulfurization involves the oxidation of sulfur compounds in diesel fuel. This process converts organic sulfur into sulfoxides and sulfones, which are more polar and can be selectively removed from the fuel. The oxidation reaction can be represented as:
R-S-R’ + O2 → R-SO-R’ + R-SO2-R’
C. Adsorptive Desulfurization Chemistry
Adsorption mechanisms in adsorptive desulfurization are based on the interaction between the sulfur compounds and the solid adsorbents. The adsorbents have specific sites that attract sulfur-containing molecules due to their electron density. As diesel fuel flows through the adsorbent bed, sulfur compounds are captured. Regeneration involves desorption, where the adsorbent is treated to release the sulfur compounds and restore its adsorption capacity.

Catalysis in Diesel Desulfurization
Catalysts play a crucial role in diesel desulfurization processes, particularly in HDS and ODS.
- Role of Catalysts
In HDS, catalysts, such as cobalt and molybdenum, provide active sites where the breaking of sulfur-carbon bonds can occur more readily. These metal catalysts promote the dissociation of hydrogen molecules, allowing them to react with sulfur compounds in diesel fuel. The catalysts also aid in the removal of sulfur from the catalyst surface, thereby ensuring continued catalytic activity.
- Catalyst Deactivation
Catalysts can become deactivated over time due to various factors, such as the accumulation of impurities, coke formation, or poisoning by certain contaminants. When a catalyst loses its activity, it needs to be regenerated or replaced to maintain the efficiency of the desulfurization process. Regeneration typically involves high-temperature treatments or chemical processes to restore the catalyst’s activity.
- Recent Advances in Catalyst Development
Continuous research and development efforts have led to innovations in catalyst design. Novel catalysts are being developed to enhance catalytic activity, selectivity, and resistance to deactivation. These advancements aim to make diesel desulfurization processes more efficient and environmentally friendly.
Environmental Benefits of Diesel Desulfurization
Reduction in Sulfur Emissions
Diesel desulfurization significantly reduces the sulfur content in diesel fuel. As a result, the combustion of low-sulfur diesel produces fewer sulfur dioxide (SO2) emissions, which are a major contributor to air pollution and acid rain. Reducing SO2 emissions improves air quality, protects human health, and mitigates environmental damage.
Improved Fuel Efficiency
Lower sulfur content in diesel fuel reduces the likelihood of sulfur-induced corrosion and fouling in engines and exhaust systems. Clean diesel fuels lead to improved engine performance, increased fuel efficiency, and extended engine life. These benefits translate to reduced fuel consumption and greenhouse gas emissions.
Compliance with Emission Standards
Regulatory bodies worldwide have implemented stringent emission standards that mandate lower sulfur levels in diesel fuel. Diesel desulfurization helps the industry meet these standards, ensuring that vehicles and industrial processes operate within environmentally responsible limits.
Contribution to Sustainable Energy
Diesel desulfurization aligns with the broader goal of transitioning to cleaner and more sustainable energy sources. By reducing the environmental impact of diesel combustion, it supports efforts to reduce greenhouse gas emissions and combat climate change.

Conclusion
Diesel desulfurization processes are critical for reducing the environmental footprint of diesel fuel. Through methods such as hydrodesulfurization, oxidative desulfurization, and adsorptive desulfurization, sulfur content in diesel fuel can be significantly reduced. The underlying chemistry and catalysis involved in these processes are key to their success, and ongoing research is driving innovations in catalyst design.
The environmental benefits of diesel desulfurization cannot be overstated. Lower sulfur emissions, improved fuel efficiency, compliance with emission standards, and the promotion of sustainable energy make diesel desulfurization a vital component of our efforts to create a cleaner, greener future. As technology continues to advance, we can expect even more effective and environmentally friendly diesel desulfurization processes to emerge, further reducing the environmental impact of diesel fuel.