What is crude oil fractional distillation
Crude oil is a complex mixture of hydrocarbons that vary widely in molecular weight and boiling point. In its raw form, crude oil isn’t directly usable. It requires specialized processing to be converted into commercially valuable products such as gasoline, diesel, jet fuel, and lubricants. This conversion process is called crude oil fractional distillation.
Fractional distillation is a thermal separation technique that works on a simple physical principle: components in crude oil are separated based on their boiling point ranges. Unlike chemical reactions, this process does not alter molecular structures. Instead, it relies on controlled heating and condensation cycles to separate crude oil into distinct fractions.
| Stage | Function |
| Crude Heating | Heats crude oil to approximately 350–400°C |
| Flash Zone (Tower Base) | Initial vaporization and start of separation |
| Column Trays/Draw-Offs | Collects different fractions by boiling point |
| Overhead Condenser | Recovers lighter vapors like LPG and naphtha |
How Crude Oil Fractional Distillation Works
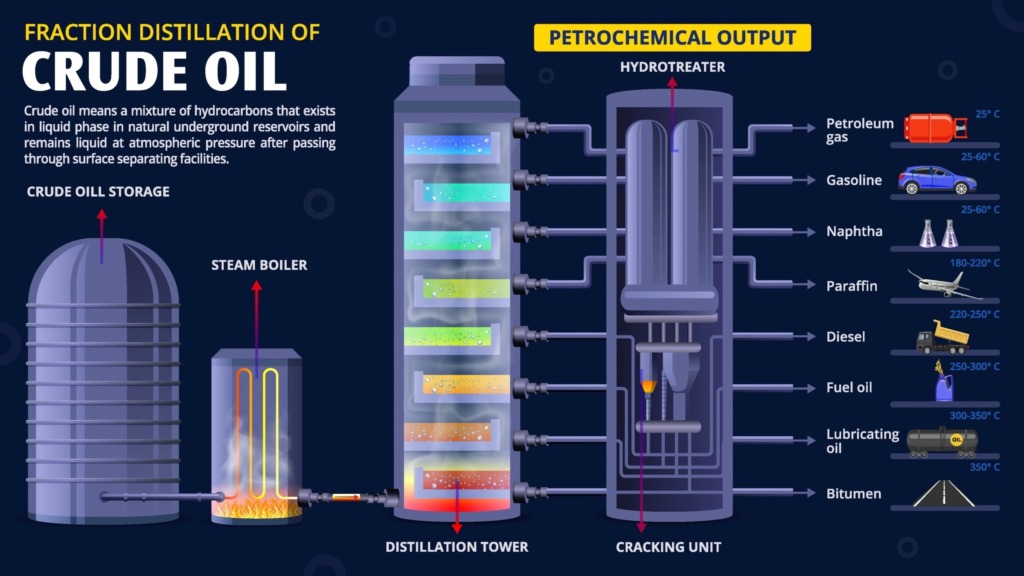
Crude oil fractional distillation is a physicochemical process based on the differences in boiling points of hydrocarbons to alter the molecular structure of the compounds. Instead, it relies on a cyclical process of vaporization and condensation to separate the components. (read more: How Crude Oil Is Separated into Fractions)
Inside the distillation tower, the temperature decreases gradually from bottom to top. As the crude oil is heated:
- Different hydrocarbon chains vaporize at specific temperatures and rise through the column.
- When they reach their condensation point, they revert to liquid form and are drawn off at various levels within the tower.
- Lighter hydrocarbons (such as methane and propane) rise to the top of the column.
- Heavier hydrocarbons (such as diesel and bitumen) condense near the bottom or remain in a liquid state.
- This principle allows crude oil to be systematically separated into a range of commercially valuable products within a single integrated unit.
Commercial Value of Crude Oil Fractional Distillation
The output of fractional distillation isn’t just a collection of chemical fractions—it represents a diverse portfolio of products essential to multiple industries.
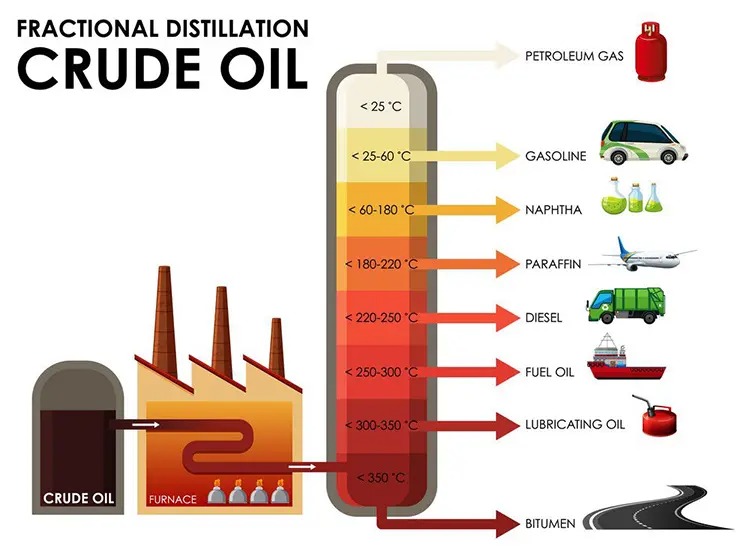
| Fraction | Boiling Range (°C) | Key Products | Core Industry Applications |
| Refinery Gases | < 30°C | LPG, propane, butane | Domestic cooking, petrochemical steam cracking |
| Naphtha | 30–180°C | Petrochemical feedstocks | Road construction, roofing, and further vacuum processing |
| Gasoline | 40–205°C | Automotive fuel | Transportation, private vehicles, fuel retail |
| Kerosene | 150–275°C | Jet fuel, heating oil | Aviation, residential and commercial heating |
| Diesel | 200–350°C | Commercial engine fuel | Plastics, solvents, and synthetic fibers |
| Lubricating Oils | 300–450°C | Engine oils, greases | Vehicle maintenance, industrial machinery lubrication |
| Fuel Oils | 350–600°C | Marine fuel, boiler fuel | Shipping vessels, industrial power boilers |
| Residuum (Bitumen) | > 600°C | Asphalt, vacuum residue | Road construction, roofing, further vacuum processing |
Summary: Why Crude Oil Fractional Distillation Matters
Fractional distillation is the foundation of refinery operations, transforming raw crude into structured, saleable products. For energy buyers and industry professionals, understanding this process is key to making informed decisions across the oil value chain—from procurement to market strategy.
Though invisible to most consumers, fractional distillation is critical because:
- Cars depend on gasoline distilled from crude oil.
- Airplanes rely on kerosene-based jet fuel.
- Factories require lubricants and petrochemicals produced from crude fractions.
- Without this process, modern life and industrial functions would face significant disruption.








